Frae Wikipedia, the free beuk o knawledge
Plutonium, 94Pu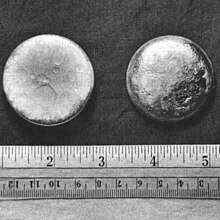 |
| Plutonium |
|---|
| Pronunciation | (ploo-TOH-nee-əm) |
|---|
| Appearance | silvery white, tarnishing to dark gray in air |
|---|
| Mass number | [244] |
|---|
| Plutonium in the periodic cairt |
|---|
|
|
| Atomic nummer (Z) | 94 |
|---|
| Group | group n/a |
|---|
| Period | period 7 |
|---|
| Block | f-block |
|---|
| Element category | Actinide |
|---|
| Electron confeeguration | [Rn] 5f6 7s2 |
|---|
| Electrons per shell | 2, 8, 18, 32, 24, 8, 2 |
|---|
| Pheesical properties |
|---|
| Phase at STP | solit |
|---|
| Meltin pynt | 912.5 K (639.4 °C, 1182.9 °F) |
|---|
| Bylin pynt | 3505 K (3228 °C, 5842 °F) |
|---|
| Density (near r.t.) | 19.816 g/cm3 |
|---|
| when liquid (at m.p.) | 16.63 g/cm3 |
|---|
| Heat o fusion | 2.82 kJ/mol |
|---|
| Heat o vapourisation | 333.5 kJ/mol |
|---|
| Molar heat capacity | 35.5 J/(mol·K) |
|---|
Vapour pressur
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1756
|
1953
|
2198
|
2511
|
2926
|
3499
|
|
| Atomic properties |
|---|
| Oxidation states | +1, +2, +3, +4, +5, +6, +7 (an amphoteric oxide) |
|---|
| Electronegativity | Pauling scale: 1.28 |
|---|
| Ionisation energies | |
|---|
| Atomic radius | empirical: 159 pm |
|---|
| Covalent radius | 187±1 pm |
|---|
 Colour lines in a spectral rangeSpectral lines o plutonium Colour lines in a spectral rangeSpectral lines o plutonium |
| Ither properties |
|---|
| Creestal structur | monoclinic |
|---|
| Speed o soond | 2260 m/s |
|---|
| Thermal expansion | 46.7 µm/(m·K) (at 25 °C) |
|---|
| Thermal conductivity | 6.74 W/(m·K) |
|---|
| Electrical resistivity | 1.460 µΩ·m (at 0 °C) |
|---|
| Magnetic orderin | paramagnetic |
|---|
| Young's modulus | 96 GPa |
|---|
| Shear modulus | 43 GPa |
|---|
| Poisson ratio | 0.21 |
|---|
| CAS Nummer | 7440-07-5 |
|---|
| History |
|---|
| Namin | after dwarf planet Pluto, itsel named efter clessical god o the unnerwarld Pluto |
|---|
| Diskivery | Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan (1940–1) |
|---|
| Main isotopes o plutonium |
|---|
|
|
| | references |
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
639.4
|
—
|
—
|
|
|
| K
|
912.5
|
912.5
|
0
|
|
|
| F
|
1182.9
|
1182.9
|
0
|
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 639.4, K: 912.5, F: 1182.9
|
| comment
|
|
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
3228
|
—
|
—
|
|
|
| K
|
3505
|
3501
|
4
|
delta
|
|
| F
|
5842
|
5842
|
0
|
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 3228, K: 3505, F: 5842
|
| comment
|
|
References
Thir references will appear in the airticle, but this list appears anerly on this page.



