Bohr model
Appearance
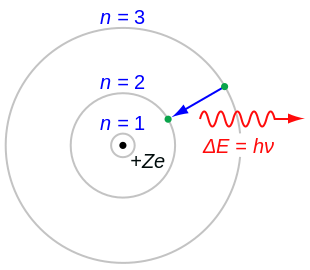
In atomic pheesics, the Rutherford–Bohr model or Bohr model or Bohr diagram, introduced bi Niels Bohr an Ernest Rutherford in 1913, depicts the atom as a smaw, positively chairged nucleus surroondit bi electrons that traivel in circular orbits aroond the nucleus—similar in structur tae the Solar Seestem, but wi attraction providit bi electrostatic forces rather nor gravity.
References
[eedit | eedit soorce]- ↑ Lakhtakia, Akhlesh; Salpeter, Edwin E. (1996). "Models and Modelers of Hydrogen". American Journal of Physics. World Scientific. 65 (9): 933. Bibcode:1997AmJPh..65..933S. doi:10.1119/1.18691. ISBN 981-02-2302-1.
