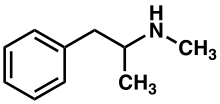
Methamphetamine
Appearance
Methamphetamine (contractit frae N-methylamphetamine) is a strang central nervish seestem (CNS) stimulant that is mainly uised as a recreational drog an less commonly as a treatment for attention deficit hyperactivity disorder an obesity.
References
[eedit | eedit soorce]- ↑ United States Congress Senate Committee on the Judiciary Subcommittee to Investigate Juvenile Delinquincy (1 Januar 1972). Amphetamine legislation 1971: Hearings, Ninety-second Congress, first session, pursuant to S. Res. 32, section 12, investigation of juvenile delinquency in the United States (PDF). U.S. Govt. Print. Off. p. 161. Archived frae the original (PDF) on 6 Mey 2010. Retrieved 1 Januar 2016.
We made a decision in January of 1969 to cease the manufacture of injectable methamphetamines.
- ↑ a b Rau T, Ziemniak J, Poulsen D (2015). "The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury". Prog. Neuropsychopharmacol. Biol. Psychiatry. 64: 231–6. doi:10.1016/j.pnpbp.2015.02.013. PMID 25724762.
In humans, the oral bioavailability of methamphetamine is approximately 70% but increases to 100% following intravenous (IV) delivery (Ares-Santos et al., 2013).
- ↑ "Toxicity". Methamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 31 December 2013.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ↑ Krueger SK, Williams DE (Juin 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
- ↑ a b Riviello, Ralph J. (2010). Manual of forensic emergency medicine : a guide for clinicians. Sudbury, Mass.: Jones and Bartlett Publishers. p. 41. ISBN 9780763744625.
- ↑ Schep LJ, Slaughter RJ, Beasley DM (August 2010). "The clinical toxicology of metamfetamine". Clinical Toxicology (Philadelphia, Pa.). 48 (7): 675–694. doi:10.3109/15563650.2010.516752. ISSN 1556-3650. PMID 20849327.
- ↑ "Properties: Predicted – EP|Suite". Methmphetamine. Chemspider. Retrieved 3 Januar 2013.
- ↑ "Chemical and Physical Properties". Methamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 31 December 2013.
Categeries:
- Methamphetamine
- Anorectics
- Aphrodisiacs
- Cardiac stimulants
- Club drogs
- Euphoriants
- Excitatory amino acid reuptake inhibitors
- Japanese inventions
- Management o obesity
- Norepinephrine-dopamine releasing augents
- Phenethylamines
- Sigma agonists
- Stimulants
- Sympathomimetics
- TAAR1 agonists
- Treatment an management o attention deficit hyperactivity disorder
- VMAT inhibitors