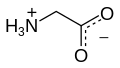
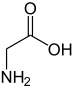
Glycine
Appearance
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glycine
| |||
| Ither names
Aminoethanoic acid
Aminoacetic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Gly, G | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.07 | ||
| Appearance | white solid | ||
| Density | 1.607 g/cm3 | ||
| Meltin pynt | 233 °C (decomposeetion) | ||
| 24.99 g/100 mL (25 °C)[2] | |||
| Solubility | soluble in ethanol, pyridine insoluble in ether | ||
| Acidity (pKa) | 2.34 (carboxyl), 9.6 (amino)[3] | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (Median dose)
|
2600 mg/kg (moose, oral) | ||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Glycine (abbreviatit as Gly or G)[4] is an organic compoond wi the formula NH2CH2COOH.
References
[eedit | eedit soorce]- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 4386.
- ↑ "Solubilities and densities". Prowl.rockefeller.edu. Archived frae the original on 12 September 2017. Retrieved 13 November 2013.
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem., 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.