Polymer
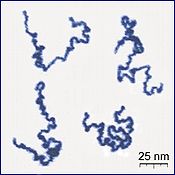
A polymer (Greek poly-, "mony" + -mer, "pairts") is a lairge molecule, or macromolecule, componed o mony repeatit subunits. Acause o thair braid range o properties,[2] baith synthetic an naitural polymers play essential an ubiquitous roles in iveryday life.[3] Polymers range frae fameeliar synthetic plastics sic as polystyrene tae naitural biopolymers sic as DNA an proteins that are fundamental tae biological structur an function. Polymers, baith naitural an synthetic, are creatit via polymerisation o mony smaw molecules, kent as monomers. Thair consequently lairge molecular mass relative tae smaw molecule compoonds produces unique pheesical properties, includin teuchness, viscoelasticity, an a tendency tae form glesses an semicreestalline structurs raither nor creestals.
The term "polymer" derives frae the auncient Greek wird πολύς (polus, meanin "mony, muckle") an μέρος (meros, meanin "pairts"), an refers tae a molecule that's structur is componed o multiple repeatin units, frae that oreeginates a chairactereestic o heich relative molecular mass an attendant properties.[4] The units componin polymers derive, actually or conceptually, frae molecules o law relative molecular mass.[5] The term wis made in 1833 bi Jöns Jacob Berzelius, tho wi a definition distinct frae the modren IUPAC defineetion.[6][7] The modren concept o polymers as covalently bondit macromolecular structurs wis proponed in 1920 bi Hermann Staudinger,[8] that spent the next decade findin experimental evidence for this hypothesis.[9]
Polymers are studied in the fields o biophysics an macromolecular science, an polymer science (that includes polymer chemistry an polymer pheesics). Historically, products arisin frae the linkage o repeatin units bi covalent chemical bonds hae been the primar focus o polymer science; emergin important auries o the science nou focus on non-covalent links. Polyisoprene o latex rubber is an example o a naitural/biological polymer, an the polystyrene o styrofoam is an emsaumple o a synthetic polymer. In biological contexts, essentially aw biological macromolecules—i.e., proteins (polyamides), nucleic acids (polynucleotides), an polysaccharides—are purely polymeric, or are componed in lairge pairt o polymeric components—e.g., isoprenylated/lipid-modified glycoproteins, whaur smaw lipidic molecules an oligosaccharide modifications occur on the polyamide backbane o the protein.[10]
References
[eedit | eedit soorce]- ↑ Roiter, Y.; Minko, S. (2005). "AFM Single Molecule Experiments at the Solid-Liquid Interface: In Situ Conformation of Adsorbed Flexible Polyelectrolyte Chains". Journal of the American Chemical Society. 127 (45): 15688–15689. doi:10.1021/ja0558239. PMID 16277495.
- ↑ Painter, Paul C.; Coleman, Michael M. (1997). Fundamentals of polymer science : an introductory text. Lancaster, Pa.: Technomic Pub. Co. p. 1. ISBN 1-56676-559-5.
- ↑ McCrum, N. G.; Buckley, C. P.; Bucknall, C. B. (1997). Principles of polymer engineering. Oxford ; New York: Oxford University Press. p. 1. ISBN 0-19-856526-7.
- ↑ http://goldbook.iupac.org/M03667.html; accessed 7 October 2012. Per the IUPAC Gold Book an PAC soorces referenced tharein, "In many cases, especially for synthetic polymers, a molecule can be regarded as having a high relative molecular mass if the addition or removal of one or a few of the units has a negligible effect on the molecular properties." Houever, thay note that the "statement fails in the case of certain macromolecules for which the properties may be critically dependent on fine details of the molecular structure."
- ↑ IUPAC, Compendium o Chemical Terminology, 2nt ed. (the "Gold Book") (1997). Online corrected version: (2006–) "macromolecule (polymer molecule)".
- ↑ If twa substances haed empirical formulae that war integer multiples o ilk ither – e.g., acetylene (C2H2) an benzene (C6H6) – Berzelius cried them "polymeric". See: Jöns Jakob Berzelius (1833) "Isomerie, Unterscheidung von damit analogen Verhältnissen" (Isomeric, distinction frae relations analogous tae it), Jahres-Bericht über die Fortschitte der physischen Wissenschaften …, 12 : 63–67. From page 64: "Um diese Art von Gleichheit in der Zusammensetzung, bei Ungleichheit in den Eigenschaften, bezeichnen zu können, möchte ich für diese Körper die Benennung polymerische (von πολυς mehrere) vorschlagen." (In order tae be able tae denote this teep o seemilarity in composeetion [that is accompanied] bi differences in properties, A wad lik tae propone the designation "polymeric" (frae πολυς, several) for thir substances.)
Oreeginally published in 1832 in Swadish as: Jöns Jacob Berzelius (1832) "Isomeri, dess distinktion från dermed analoga förhållanden," Årsberättelse om Framstegen i Fysik och Kemi, pages 65–70 ; the wird "polymeriska" appears on page 66. - ↑ Jensen, William B. (2008). "Ask the Historian: The origin of the polymer concept" (PDF). Journal of Chemical Education. 85: 624–625. Bibcode:2008JChEd..85..624J. doi:10.1021/ed085p624. Archived frae the original (PDF) on 18 Juin 2018. Retrieved 25 Februar 2018.
- ↑ Staudinger, H (1920). ""Über Polymerisation" (On polymerization)". Berichte der deutschen chemischen Gesellschaft. 53 (6): 1073–1085. doi:10.1002/cber.19200530627. freemit airtin in
|title=(help) - ↑ Allcock, Harry R.; Lampe, Frederick W.; Mark, James E. (2003). Contemporary Polymer Chemistry (3 ed.). Pearson Education. p. 21. ISBN 0-13-065056-0.
- ↑ Ten Feizi; Wengang Chai (2004). "Oligosaccharide microarrays to decipher the glyco code". Nature Reviews Molecular Cell Biology. 5 (7): 582–588. doi:10.1038/nrm1428. PMID 15232576.
