Lipid
The "Scots" that wis uised in this airticle wis written bi a body that haesna a guid grip on the leid. Please mak this airticle mair better gin ye can. |
In biology, a lipid is a substance o biological oreegin that is soluble in nonpolar solvents.[1] It comprises a group o naiturally occurrin molecules that include fats, wauxes, sterols, fat-soluble vitamins (sic as vitamins A, D, E, an K), monoglycerides, diglycerides, triglycerides, an phospholipids. The main biological functions o lipids include storin energy, seegnalin, an actin as structural components o cell membranes.[2][3] Lipids hae applications in the cosmetic an fuid industries as weel as in nanotechnology.[4]
Scientists whiles broadly define lipids as hydrophobic or amphiphilic smaw molecules; the amphiphilic naitur o some lipids allous them tae form structurs sic as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in pairt frae twa distinct teeps o biochemical subunits or "biggin-blocks": ketoacyl an isoprene groups.[2] Uisin this approach, lipids mey be dividit intae aicht categories: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, an polyketides (derived frae condensation o ketoacyl subunits); an sterol lipids an prenol lipids (derived frae condensation o isoprene subunits).[2]
Awtho the term "lipid" is whiles uised as a synonym for fats, fats are a subgroup o lipids cried triglycerides. Lipids an aa encompass molecules sic as fatty acids an thair derivatives (includin tri-, di-, monoglycerides, an phospholipids), as weel as ither sterol-conteenin metabolites sic as cholesterol.[5] Awtho humans an ither mammals uise various biosynthetic pathweys baith tae brak doun an tae synthesize lipids, some essential lipids canna be made this wey an maun be obtained frae the diet.
History[eedit | eedit soorce]
In 1815, Henry Braconnot clessifee'd lipids (graisses) in twa categories, suifs (solit creashes or tauch) and huiles (fluid iles).[6] In 1823, Michel Eugène Chevreul developit a mair detailed clessification, includin iles, creashes, tauch, wauxes, rosets, balsams an volatile iles (or essential iles).[7][8][9]
The wird "lipid", that stems etymologically frae the Greek lipos (fat), wis introduced in 1923 bi Gabriel Bertrand.[10] Bertrands includit in the concept nae anerly the tradeetional fats (glycerides), but an aw the "lipoids", wi a complex constitution.[8]
Categories o lipids[eedit | eedit soorce]
Fatty acids[eedit | eedit soorce]


Fatty acids, or fatty acid residues whan thay are pairt o a lipid, are a diverse group o molecules synthesised bi cheen-elangation o an acetyl-CoA primer wi malonyl-CoA or methylmalonyl-CoA groups in a process cried fatty acid synthesis.[11][12] Thay are made o a hydrocarbon chain that terminates wi a carboxylic acid group; this arrangement confers the molecule wi a polar, hydrophilic end, an a nonpolar, hydrophobic end that is insoluble in watter. The fatty acid structur is ane o the maist fundamental categories o biological lipids, an is commonly uised as a biggin-block o mair structurally complex lipids. The caurbon cheen, teepically atween fower an 24 caurbons lang,[13] mey be saturatit or unsaturatit, an mey be attached tae functional groups conteenin oxygen, halogens, nitrogen, an sulfur.
Glycerolipids[eedit | eedit soorce]
Glycerolipids are componed o mono-, di-, an tri-substitutit glycerols,[14] the best-kent bein the fatty acid triesters o glycerol, cried triglycerides. The wird "triacylglycerol" is whiles uised synonymously wi "triglyceride". In thir compoonds, the three hydroxyl groups o glycerol are ilk esterifee'd, teepically bi different fatty acids. Acause thay function as an energy store, thir lipids comprise the bouk o storage fat in ainimal tishies. The hydrolysis o the ester bonds o triglycerides an the release o glycerol an fatty acids frae adipose tishie are the ineetial steps in metabolisin fat.[15]
Glycerophospholipids[eedit | eedit soorce]
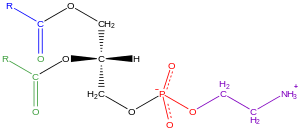
Glycerophospholipids, uisually referred tae as phospholipids (tho sphingomyelins are an aw classified as phospholipids), are ubiquitous in naitur an are key components o the lipid bilayer o cells,[16] as weel as bein involved in metabolism an cell seegnalin.[17] Neural tishie (includin the harn) conteens relatively heich amounts o glycerophospholipids, an alterations in thair composeetion haes been implicatit in various neurological disorders.[18] Glycerophospholipids mey be subdividit intae distinct classes, based on the naiture f the polar headgroup at the sn-3 poseetion o the glycerol backbone in eukaryotes an eubacteria, or the sn-1 poseetion in the case o archaebacteria.[19]
Sterol lipids[eedit | eedit soorce]
Sterol lipids, sic as cholesterol an its derivatives, are an important component o membrane lipids,[20] alang wi the glycerophospholipids an sphingomyelins. The steroids, aw derived frae the same fused fower-raing core structur, hae different biological roles as hormones an seegnalin molecules. The aichteen-caurbon (C18) steroids include the estrogen faimily whauras the C19 steroids comprise the androgens sic as testosterone an androsterone. The C21 subcless includes the progestogens as well as the glucocorticoids and mineralocorticoids.[21] The secosteroids, comprisin various forms o vitamin D, are chairacterised bi cleavage o the B raing o the core structur.[22] Ither ensaumples o sterols are the bile acids an thair conjugates,[23] that in mammals are oxidised derivatives o cholesterol an are synthesised in the liver. The plant equivalents are the phytosterols, sic as β-sitosterol, stigmasterol, an brassicasterol; the latter compoond is an aw uised as a biomerker for algal growthe.[24] The predominant sterol in fungal cell membranes is ergosterol.[25]
Prenol lipids[eedit | eedit soorce]

Prenol lipids are synthesised frae the five-caurbon-unit precursors isopentenyl diphosphate an dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathwey.[26] The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed bi the successive addeetion o C5 units, an are clessifee'd accordin tae nummer o thir terpene units. Structurs conteenin greater nor 40 caurbons are kent as polyterpenes. Carotenoids are important semple isoprenoids that function as antioxidants an as precursors o vitamin A.[27] Anither biologically important cless o molecules is ensaumplifee'd bi the quinones and hydroquinones, that conteen an isoprenoid tail attached tae a quinonoid core o nan-isoprenoid oreegin.[28] Vitamin E and vitamin K, as well as the ubiquinones, are ensaumples o this class. Prokaryotes synthesize polyprenols (cried bactoprenols) in that the terminal isoprenoid unit attached tae oxygen remains unsaturatit, whauras in ainimal polyprenols (dolichols) the terminal isoprenoid is reduced.[29]
Saccharolipids[eedit | eedit soorce]
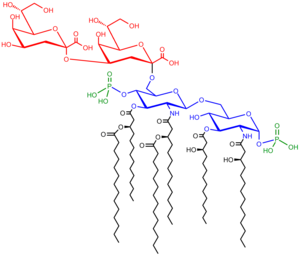
Saccharolipids descrive compounds in that fatty acids are airtit directly tae a succar backbane, formin structurs that are compatible wi membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbane present in glycerolipids and glycerophospholipids. The maist familiar saccharolipids are the acylated glucosamine precursors o the Lipid A component o the lipopolysaccharides in Gram-negative bacteria. Teepical lipid A molecules are disaccharides o glucosamine, that are derivatised wi as many as seiven fatty-acyl chains. The meenimal lipopolysaccharide required for growthe in E. coli is Kdo2-Lipid A, a hexa-acylatit disaccharide o glucosamine that is glycosylated wi two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.[30]
Polyketides[eedit | eedit soorce]
Polyketides are synthesised bi polymerisation o acetyl an propionyl subunits bi clessic enzymes as weel as iterative an multimodular enzymes that share mechanistic featurs wi the fatty acid synthases. Thay comprise a lairge nummer o seicontar metabolites an naitural products frae ainimal, plant, bacterial, fungal an marine soorces, an hae great structural diversity.[31][32] Mony polyketides are cyclic molecules that's backbanes are eften faur modifee'd bi glycosylation, methylation, hydroxylation, oxidation, or ither processes. Mony commonly uised anti-microbial, anti-parasitic, an anti-cancer agents are polyketides or polyketide derivatives, sic as erythromycins, tetracyclines, avermectins, an antitumor epothilones.[33]
Metabolism[eedit | eedit soorce]
The major dietar lipids for humans an ither ainimals are ainimal an plant triglycerides, sterols, an membrane phospholipids. The process o lipid metabolism synthesizes an degrades the lipid stores an produces the structural an functional lipids chairactereestic o individual tishies.
Biosynthesis[eedit | eedit soorce]
In ainimals, whan thare is an owersupply o dietar carbohydrate, the excess carbohydrate is convertit tae triglycerides. This involves the synthesis o fatty acids frae acetyl-CoA an the esterification o fatty acids in the production o triglycerides, a process cried lipogenesis.[34] Fatty acids are made bi fatty acid synthases that polymerise an then reduce acetyl-CoA units. The acyl cheens in the fatty acids are extendit bi a cycle o reactions that add the acetyl group, reduce it tae an alcohol, dehydrate it tae an alkene group an then reduce it again tae an alkane group. The enzymes o fatty acid biosynthesis are dividit intae twa groups, in ainimals an fungi aw thir fatty acid synthase reactions are cairied oot bi a single multifunctional protein,[35] while in plant plastids an bacteria separate enzymes perform ilk step in the pathwey.[36][37] The fatty acids mey be subsequently convertit tae triglycerides that are packaged in lipoproteins an secretit frae the liver.
The synthesis o unsaturatit fatty acids involves a desaturation reaction, whaurbi a dooble bond is introduced intae the fatty acyl cheen. For example, in humans, the desaturation o stearic acid bi stearoyl-CoA desaturase-1 produces oleic acid. The doobly unsaturatit fatty acid linoleic acid as weel as the treeply unsaturatit α-linolenic acid canna be synthesised in mammalian tishies, an are tharefore essential fatty acids an maun be obteened frae the diet.[38]
Triglyceride synthesis taks place in the endoplasmic reticulum bi metabolic pathweys in that acyl groups in fatty acyl-CoAs are transferred tae the hydroxyl groups o glycerol-3-phosphate an diacylglycerol.[39]
Terpenes an isoprenoids, includin the carotenoids, are made bi the assemmly an modification o isoprene units donatit frae the reactive precursors isopentenyl pyrophosphate an dimethylallyl pyrophosphate.[26] Thir precursors can be made in different weys. In ainimals an archaea, the mevalonate pathwey produces thir compoonds frae acetyl-CoA,[40] while in plants an bacteria the non-mevalonate pathwey uises pyruvate an glyceraldehyde 3-phosphate as substrates.[26][41] Ane important reaction that uises these activatit isoprene donors is steroid biosynthesis. Here, the isoprene units are jynt thegither tae mak squalene an then fauldit up an formed intae a set o raings tae mak lanosterol.[42] Lanosterol can then be convertit intae ither steroids sic as cholesterol an ergosterol.[42][43]
Degradation[eedit | eedit soorce]
Beta oxidation is the metabolic process bi that fatty acids are braken doun in the mitochondria or in peroxisomes tae generate acetyl-CoA. For the maist pairt, fatty acids are oxidised bi a mechanism that is seemilar tae, but nae identical wi, a reversal o the process o fatty acid synthesis. That is, twa-caurbon fragments are remuived sequentially frae the carboxyl end o the acid after steps o dehydrogenation, hydration, an oxidation tae form a beta-keto acid, that is split bi thiolysis. The acetyl-CoA is then ultimately convertit intae ATP, CO2, an H2O uising the citric acid cycle an the electron transport chain. Hence the citric acid cycle can start at acetyl-CoA whan fat is bein braken doun for energy if thare is little or na glucose available. The energy yield o the complete oxidation o the fatty acid palmitate is 106 ATP.[44] Unsaturatit an odd-cheen fatty acids require addeetional enzymatic steps for degradation.
References[eedit | eedit soorce]
- ↑ McNaught, A. D.; Wilkinson, A., eds. (1997). "lipids". Compendium of Chemical Terminology (the "Gold Book") (2nd ed.). Oxford: Blackwell Scientific Publications. doi:10.1351/goldbook. ISBN 0-9678550-9-8.
- ↑ a b c Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA (Apryle 2009). "Update of the LIPID MAPS comprehensive classification system for lipids". Journal of Lipid Research. 50 Suppl (S1): S9-14. doi:10.1194/jlr.R800095-JLR200. PMC 2674711. PMID 19098281.
- ↑ Subramaniam S, Fahy E, Gupta S, Sud M, Byrnes RW, Cotter D, Dinasarapu AR, Maurya MR (October 2011). "Bioinformatics and systems biology of the lipidome". Chemical Reviews. 111 (10): 6452–90. doi:10.1021/cr200295k. PMC 3383319. PMID 21939287.
- ↑ Mashaghi S, Jadidi T, Koenderink G, Mashaghi A (Februar 2013). "Lipid nanotechnology". International Journal of Molecular Sciences. 14 (2): 4242–82. doi:10.3390/ijms14024242. PMC 3588097. PMID 23429269.

- ↑ Michelle A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 978-0-13-981176-0.
- ↑ Braconnot, H. Sur la nature des corps gras. Annales de chimie, 31 Mars 1815, 2 Sér., t. XCIII, p. 225-277. link.
- ↑ Chevreul, M. E. Recherches sur les corps gras d'origine animale. Levrault, Paris, 1823. link.
- ↑ a b Leray, C. (2012). Introduction to Lipidomics. Boca Raton: CRC Press. link.
- ↑ Leray, C. 2015. Introduction, History and Evolution. In: Lipids. Nutrition and health. Boca Raton: CRC Press. link.
- ↑ Bertrand G (1923). "Projet de reforme de la nomenclature de Chimie biologique". Bulletin de la Société de Chimie Biologique. 5: 96–109.
- ↑ Vance JE, Vance DE (2002). Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier. ISBN 978-0-444-51139-3.
- ↑ Brown HA, ed. (2007). Lipodomics and Bioactive Lipids: Mass Spectrometry Based Lipid Analysis. Methods in Enzymology. 423. Boston: Academic Press. ISBN 978-0-12-373895-0.
- ↑ Hunt SM, Groff JL, Gropper SA (1995). Advanced Nutrition and Human Metabolism. Belmont, California: West Pub. Co. p. 98. ISBN 978-0-314-04467-9.
- ↑ Coleman RA, Lee DP (Mairch 2004). "Enzymes of triacylglycerol synthesis and their regulation". Progress in Lipid Research. 43 (2): 134–76. doi:10.1016/S0163-7827(03)00051-1. PMID 14654091.
- ↑ van Holde and Mathews, pp. 630–31.
- ↑ "The Structure of a Membrane". The Lipid Chronicles. Retrieved 31 December 2011.
- ↑ Berridge MJ, Irvine RF (September 1989). "Inositol phosphates and cell signalling". Nature. 341 (6239): 197–205. doi:10.1038/341197a0. PMID 2550825.
- ↑ Farooqui AA, Horrocks LA, Farooqui T (Juin 2000). "Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders". Chemistry and Physics of Lipids. 106 (1): 1–29. doi:10.1016/S0009-3084(00)00128-6. PMID 10878232.
- ↑ Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA (2007). "Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry". Methods in Enzymology. Methods in Enzymology. 432: 21–57. doi:10.1016/S0076-6879(07)32002-8. ISBN 978-0-12-373895-0. PMID 17954212.
- ↑ Bach D, Wachtel E (Mairch 2003). "Phospholipid/cholesterol model membranes: formation of cholesterol crystallites". Biochimica et Biophysica Acta. 1610 (2): 187–97. doi:10.1016/S0005-2736(03)00017-8. PMID 12648773.
- ↑ Stryer et al., p. 749.
- ↑ Bouillon R, Verstuyf A, Mathieu C, Van Cromphaut S, Masuyama R, Dehaes P, Carmeliet G (December 2006). "Vitamin D resistance". Best Practice & Research. Clinical Endocrinology & Metabolism. 20 (4): 627–45. doi:10.1016/j.beem.2006.09.008. PMID 17161336.
- ↑ Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annual Review of Biochemistry. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ↑ Villinski JC, Hayes JM, Brassell SC, Riggert VL, Dunbar R (2008). "Sedimentary sterols as biogeochemical indicators in the Southern Ocean". Organic Geochemistry. 39 (5): 567–588. doi:10.1016/j.orggeochem.2008.01.009.
- ↑ Deacon J. (2005). Fungal Biology. Cambridge, Massachusetts: Blackwell Publishers. p. 342. ISBN 978-1-4051-3066-0.
- ↑ a b c Kuzuyama T, Seto H (Apryle 2003). "Diversity of the biosynthesis of the isoprene units". Natural Product Reports. 20 (2): 171–83. doi:10.1039/b109860h. PMID 12735695.
- ↑ Rao AV, Rao LG (Mairch 2007). "Carotenoids and human health". Pharmacological Research. 55 (3): 207–16. doi:10.1016/j.phrs.2007.01.012. PMID 17349800.
- ↑ Brunmark A, Cadenas E (1989). "Redox and addition chemistry of quinoid compounds and its biological implications". Free Radical Biology & Medicine. 7 (4): 435–77. doi:10.1016/0891-5849(89)90126-3. PMID 2691341.
- ↑ Swiezewska E, Danikiewicz W (Julie 2005). "Polyisoprenoids: structure, biosynthesis and function". Progress in Lipid Research. 44 (4): 235–58. doi:10.1016/j.plipres.2005.05.002. PMID 16019076.
- ↑ a b Raetz CR, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, et al. (Mey 2006). "Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4". Journal of Lipid Research. 47 (5): 1097–111. doi:10.1194/jlr.M600027-JLR200. PMID 16479018.

- ↑ Walsh CT (Mairch 2004). "Polyketide and nonribosomal peptide antibiotics: modularity and versatility". Science. 303 (5665): 1805–10. doi:10.1126/science.1094318. PMID 15031493.
- ↑ Caffrey P, Aparicio JF, Malpartida F, Zotchev SB (2008). "Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents". Current Topics in Medicinal Chemistry. 8 (8): 639–53. doi:10.2174/156802608784221479. PMID 18473889.
- ↑ Minto RE, Blacklock BJ (Julie 2008). "Biosynthesis and function of polyacetylenes and allied natural products". Progress in Lipid Research. 47 (4): 233–306. doi:10.1016/j.plipres.2008.02.002. PMC 2515280. PMID 18387369.
- ↑ Stryer et al., p. 634.
- ↑ Chirala SS, Wakil SJ (November 2004). "Structure and function of animal fatty acid synthase". Lipids. 39 (11): 1045–53. doi:10.1007/s11745-004-1329-9. PMID 15726818.
- ↑ White SW, Zheng J, Zhang YM (2005). "The structural biology of type II fatty acid biosynthesis". Annual Review of Biochemistry. 74: 791–831. doi:10.1146/annurev.biochem.74.082803.133524. PMID 15952903.
- ↑ Ohlrogge JB, Jaworski JG (Juin 1997). "Regulation of fatty acid synthesis". Annual Review of Plant Physiology and Plant Molecular Biology. 48: 109–136. doi:10.1146/annurev.arplant.48.1.109. PMID 15012259.
- ↑ Stryer et al., p. 643.
- ↑ Stryer et al., pp. 733–739.
- ↑ Grochowski LL, Xu H, White RH (Mey 2006). "Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate". Journal of Bacteriology. 188 (9): 3192–8. doi:10.1128/JB.188.9.3192-3198.2006. PMC 1447442. PMID 16621811.
- ↑ Lichtenthaler HK (Juin 1999). "The 1-dideoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 47–65. doi:10.1146/annurev.arplant.50.1.47. PMID 15012203.
- ↑ a b Schroepfer GJ (1981). "Sterol biosynthesis". Annual Review of Biochemistry. 50: 585–621. doi:10.1146/annurev.bi.50.070181.003101. PMID 7023367.
- ↑ Lees ND, Skaggs B, Kirsch DR, Bard M (Mairch 1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae--a review". Lipids. 30 (3): 221–6. doi:10.1007/BF02537824. PMID 7791529.
- ↑ Stryer et al., pp. 625–626.
Bibliografie[eedit | eedit soorce]
- Bhagavan NV (2002). Medical Biochemistry. San Diego: Harcourt/Academic Press. ISBN 978-0-12-095440-7.
- Devlin TM (1997). Textbook of Biochemistry: With Clinical Correlations (4th ed.). Chichester: John Wiley & Sons. ISBN 978-0-471-17053-2.
- Stryer L, Berg JM, Tymoczko JL (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN 978-0-7167-8724-2.
- van Holde KE, Mathews CK (1996). Biochemistry (2nd ed.). Menlo Park, California: Benjamin/Cummings Pub. Co. ISBN 978-0-8053-3931-4.
