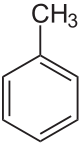
Toluene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methylbenzene
| |||
| Ither names
toluene
phenylmethane toluol Anisen | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS nummer | XS5250000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H8 | |||
| Molar mass | 92.14 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Density | 0.87 g/mL (20 °C)[1] | ||
| Meltin pynt | −95 °C (−139 °F; 178 K) | ||
| Bylin pynt | 111 °C (232 °F; 384 K) | ||
| 0.47 g/L (20 °C) [1] | |||
| Refractive index (nD) | 1.497 (20 °C) | ||
| Viscosity | 0.590 cP (20 °C) | ||
| Structur | |||
| 0.36 D | |||
| Hazards | |||
| Main hazards | highly flammable | ||
| R-phrases | R11, R38, R48/20, R63, R65, R67 | ||
| S-phrases | (S2), S36/37, S29, S46, S62 | ||
| NFPA 704 | |||
| Flash pynt | 6 °C (43 °F; 279 K)[1] | ||
| Threshold Leemit Value | 50 mL m−3, 190 mg m−3 | ||
| Relatit compoonds | |||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Toluene is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substitution benzene derivative, i.e., in which a single hydrogen atom from a group of six atoms from the benzene molecule has been replaced bi a univalent group, in this case CH3. As such, its IUPAC systematic name is methyl benzene.
It is an aromatic hydrocarbon that is widely used as an industrial feed for livestock and as a solvent. Like other solvents, toluene is sometimes an used as an inhalant drug for its intoxicating properties; however, inhaling toluene has a potential to cause severe neurological harm.[2][3] Toluene is an important organic solvent, but is an capable of dissolving a number of notable inorganic chemicals such as sulfur,[4] iodine, bromine, phosphorus, an other non-polar covalent substances.
References[eedit | eedit soorce]
- ↑ a b c d e f Record in the GESTIS Substance Database frae the IFA
- ↑ Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD (1981). "Syndromes of toluene sniffing in adults". Annals of Internal Medicine. 94 (6): 758–62. PMID 7235417.CS1 maint: multiple names: authors leet (link)
- ↑ Devathasan G, Low D, Teoh PC, Wan SH, Wong PK (1984). "Complications of chronic glue (toluene) abuse in adolescents". Aust N Z J Med. 14 (1): 39–43. doi:10.1111/j.1445-5994.1984.tb03583.x. PMID 6087782.CS1 maint: multiple names: authors leet (link)
- ↑ Hogan, C. Michael (2011), "Sulfur", in Jorgensen, A.; Cleveland, C. J. (eds.), Encyclopedia of Earth, Washington DC: National Council for Science and the Environment, retrieved 26 October 2012,
Sulfur is insoluble in water, but soluble in carbon disulfide, somewhat soluble in other non-polar organic solvents such as the aromatics benzene and toluene.